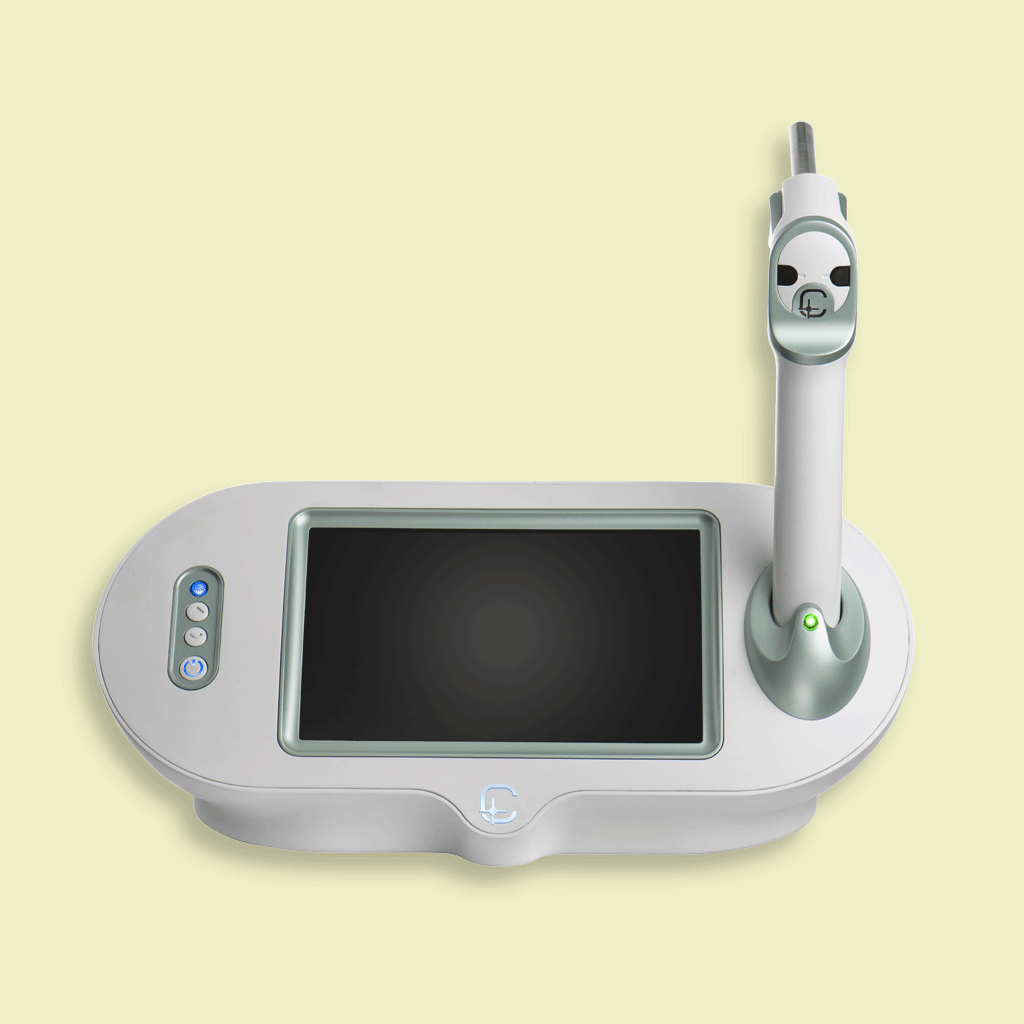
One of the company’s key products is the CDP or Cancer Diagnosis Probe, which detects tumor margins during breast cancer surgery. The CDP device, which is the first of its kind in the world with the license number 23212882 and the license of the Ministry of Health and with the tariff code 100747(the code assigned in the tariff book 1403: 5 professional components and 20 technical components), is introduced as a surgical assistant tool in breast cancer and can be used as a complementary aid system in places where there is frozen section pathology, and as a surgical assist system in places where there is no frozen section pathology. The CDP system is based on the detection of released ROS (Reactive Oxygen Species) during the metabolism of cancer cells. The system reports to the surgeon with a few millimeters of marginal accuracy each area of the internal margin of the body suspected of cancer during surgery with the help of a needle sensor with pathology calibration and has a clinical diagnostic classification that is proportional between the pathologic results of the tested tissues and the CDP response peaks, based on the pathology system classification of Ductal Intraepithelial Neoplasia (DIN), Lobular Intraepithelial Neoplasia (LIN) and Fibro Epithelial Lesion (FEL) (according to the latest reported revisions). Diagnostic results of the device, calibrated based on the pathology, appear on its display in the form of negative, suspicious and positive responses within 15 seconds. In numerous clinical trials conducted with CDP, the technology was able to detect about 30% of contaminated margins in patients even despite frozen section and permanent pathology, which was not detected by the conventional pathology method. So if CDP is not used, about 30% of patients have contaminated margins inside their bodies, which refers to the effective role of CDP as a surgeon’s aid tool during breast cancer surgery to prevent local recurrence. It should be noted that the sensitivity and specificity of the device are 93% and 91%, respectively. The CDP system is currently being used in 15 surgical centers and hospitals in the country.
The results of this system have been published in several patents in the US Patent Office, as well as in several ISI papers in the following international journals:
Published granted USA Patents: US 11,179,076 B2, US 10,786,188 B1, US 11,179,077 B2, US 11,181,499 B2, US 11806140 B2
Published ISI Journals: bioengineering and translational medicine: https://doi.org/10.1002/btm2.10236, International Journal of Medical Robotics and Computer Assisted Surgery: https://doi.org/10.1002/rcs.2335, Biosensors and Bioelectronics: https://doi.org/10.1016/j.bios.2020.112209, Cancer medicine: https://doi.org/10.1002/cam4.4503, Biosensors and Bioelectronics: https://doi.org/10.1016/j.bios.2020.112421, Medical Physics: https://doi.org/10.1002/mp.15481